Testing Number of Montipora Larvae Needed for DNA/RNA Extraction
Larvae from Biomineralization Project
Posted orginally by Maggie Schedl
Materials:
- Using Zymo Duet DNA/RNA Extraction Kit HERE**
- Thermoixer
- Centrifuge capable of 16,000 rcf spin (Eppendorf)**
Make sure ethanol has been added to the wash buffer, and that enzymes have been re-hydrated before starting
Sample Preparation
- Take sample tube with larvae 1 at a time out of the -80 to minimize amount of thawing
- Add 300µl DNA/RNA shield directly to the sample tube
- Record tube number
Samples
| Sample # | Date Collected | Type | How Many | Extracter |
|---|---|---|---|---|
| 395 | 6/26/2018 | larvae | 15 | Maggie |
| 386 | 6/26/2018 | larvae | 3 | Maggie |
| 389 | 6/26/2018 | larvae | 5 | Erin |
| 398 | 6/26/2018 | larvae | 50 | Erin |
- Add 30µl of PK digestion buffer to each sample tube
- Add 15µl Proteinase K to each sample tube
- Vortex and spin down sample tubes
- Place in Thermoixer for 1 hour at 55 degrees C, shaking at 1100 rpm
- After digestion proceed with DNA and RNA Extraction
DNA Extraction
- Set up yellow DNA spin columns and collection tubes, label appropriately
- Warm elution liquids to 70 degrees C (10mM Tris HCl pH. 8.0 and RNase free water)
- Add equal volume (345µl) DNA/RNA lysis buffer to each sample tube
- Finger flick to mix tubes
- Add 700µl (total volume) of sample gently to the yellow DNA spin column
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Important Save the flow through from this step: transfer to a new 1.5mL tube labeled for RNA
- Add 400µl DNA/RNA Prep Buffer gently to the yellow DNA spin columns
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Discard flow through (Zymo kit waste)
- Add 700µl DNA/RNA Wash Buffer gently to the yellow DNA spin columns
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Discard flow through (Zymo kit waste)
- Add 400µl DNA/RNA Wash Buffer genetly to the yellow DNA spin columns
- Centrifuge at 16,000 rcf (g) for 2 minutes
- Discard flow through (Zymo kit waste)
- Transfer yellow columns to new 1.5mL microcentrifuge tubes
- Add 50µl warmed 10mM Tris HCl to each yellow DNA column by dripping slowly directly on the filer
- Incubate at room temp for 5 minutes
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Repeat steps 18-20 for a final elution volume of 100µl
- Label tubes, store at 4 degrees C if quantifying the same day or the next, if waiting longer store in -20
RNA Extraction
- Add equal volume (700µl) 100% EtOH to the 1.5mL tubes labeled for RNA containing the original yellow column flow through
- Vortex and spin down to mix
- Add 700µl of that liquid to the green RNA spin columns
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Discard flow through (Zymo kit waste)
- Add 700µl to the green RNA spin columns (the rest from the 1.5mL RNA tubes)
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Discard flow through (Zymo kit waste)
- Add 400µl DNA/RNA Wash Buffer gently to each green RNA column
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Discard flow through (Zymo kit waste)
- Make DNase I treatment master mix:
- 75µl DNA Digestion buffer x # of samples
- 5µl DNase I x # of samples
- Add 80µl DNase I treatment master mix directly to the filter of the green RNA columns
- Incubate at room temp for 15 minutes
- Add 400µl DNA/RNA Prep Buffer gently to each column
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Discard flow through (Zymo kit waste)
- Add 700µl DNA/RNA Wash Buffer gently to the yellow DNA spin columns
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Discard flow through (Zymo kit waste)
- Add 400µl DNA/RNA Wash Buffer genetly to the yellow DNA spin columns
- Centrifuge at 16,000 rcf (g) for 2 minutes
- Discard flow through (Zymo kit waste)
- Transfer green columns to new 1.5mL microcentrifuge tubes
- Add 50µl warmed DNase/RNase free water to each green RNA column by dripping slowly directly on the filer
- Incubate at room temp for 5 minutes
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Repeat steps 25-27 for a final elution volume of 100µl
- Label 1.5mL tubes on ice afterwards, and aliquot 5µl into PCR strip tubes to save for Qubit and Tape Station to avoid freeze-thaw of your stock sample
- Store all tubes in the -80
Qubit
- Using Broad Range dsDNA and Broad Range RNA kits
- A working stock solution was made of 199µl * n buffer + n µl Quant-IT reagent
- n is # of samples + 2 + %error (usually % error was less than 1%)
- 10µl of each standard was used to create the standard curve and 1µl of each sample was used in quantification
- All quantifications are in ng/µl
- All quantifications were taken twice
| Sample | DNA Standard 1 (RFU) | DNA Standard 2 (RFU) | DNA 1 (ng/µl) | DNA 2 (ng/µl) | Average DNA | RNA Standard 1 (RFU) | RNA Standard 2 (RFU) | RNA 1 (ng/µl) | RNA 2 (ng/ul) | Average RNA |
|---|---|---|---|---|---|---|---|---|---|---|
| 395 | 198.9 | 21557 | 3.04 | 3.04 | 3.04 | 386 | 11014 | too low | - | - |
| 386 | 198.9 | 21557 | too low | - | - | 386 | 11014 | too low | - | - |
| 389 | 198.9 | 21557 | too low | - | - | 386 | 11014 | too low | - | - |
| 398 | 198.9 | 21557 | 9.78 | 9.70 | 9.74 | 386 | 11014 | 15.2 | 14.4 | 14.8 |
Tape Station
- Follow RNA Tape Station protocol here (write protocol)
- Only analyzing sample 398 because it is the only one with RNA
Results
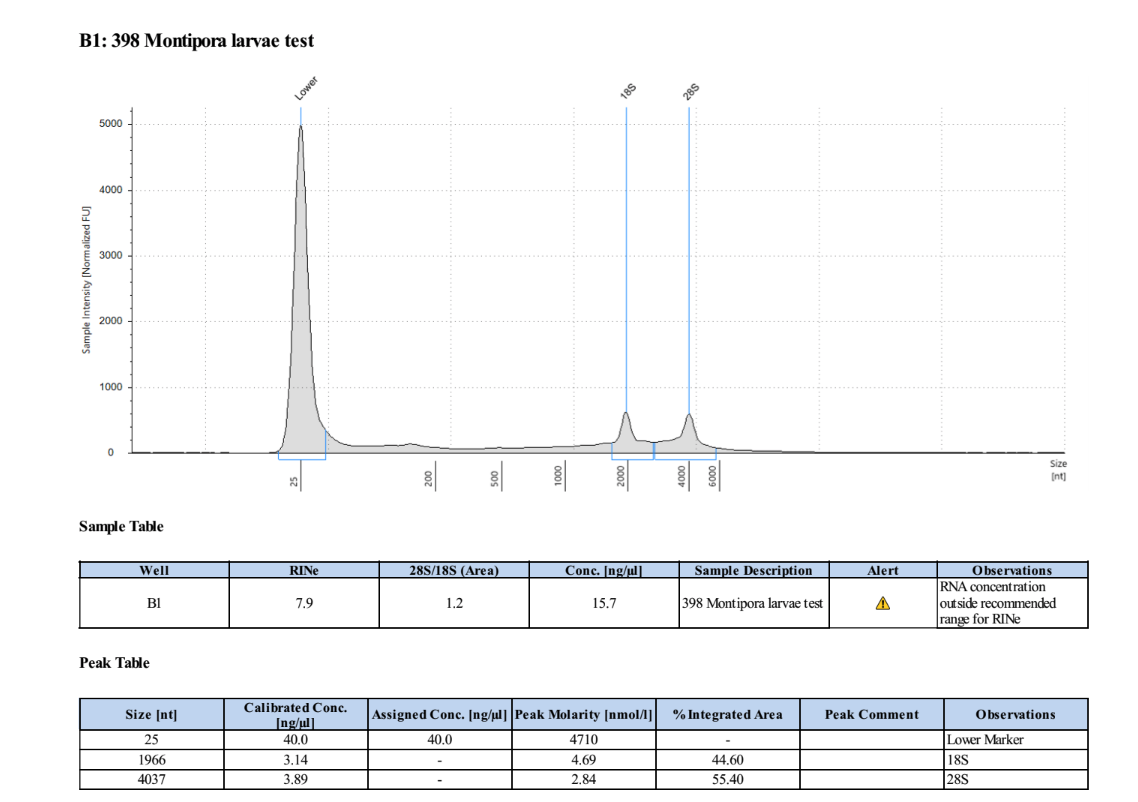
2-25-19 More Test Extractions
Samples
| Sample # | Date Collected | Type | How Many |
|---|---|---|---|
| 404 | 6/26/2018 | larvae | 100 |
| 401 | 6/26/2018 | larvae | 30 |
| 119 | 6/13/2018 | eggs | 20 |
| 102 | 6/13/2018 | bundles | 20 |
| 392 | 6/26/2018 | larvae | 10 |
Followed same extraction protocol as above
Quibit
| Sample | DNA Standard 1 (RFU) | DNA Standard 2 (RFU) | DNA 1 (ng/µl) | DNA 2 (ng/µl) | Average DNA | RNA Standard 1 (RFU) | RNA Standard 2 (RFU) | RNA 1 (ng/µl) | RNA 2 (ng/ul) | Average RNA |
|---|---|---|---|---|---|---|---|---|---|---|
| 404 | 196 | 21470 | 12.9 | 12.7 | 12.8 | 393 | 10798 | 46 | 45.8 | 45.9 |
| 401 | 196 | 21470 | 21 | 21.4 | 21.2 | 393 | 10798 | 27.8 | 27.6 | 27.7 |
| 119 | 196 | 21470 | too low | - | - | 393 | 10798 | 101 | 101 | 101 |
| 102 | 196 | 21470 | 70.6 | 71.6 | 71.1 | 393 | 10798 | too low | - | - |
| 392 | 196 | 21470 | 4.32 | 4.28 | 4.3 | 393 | 10798 | too low | - | - |
Tape Station Results
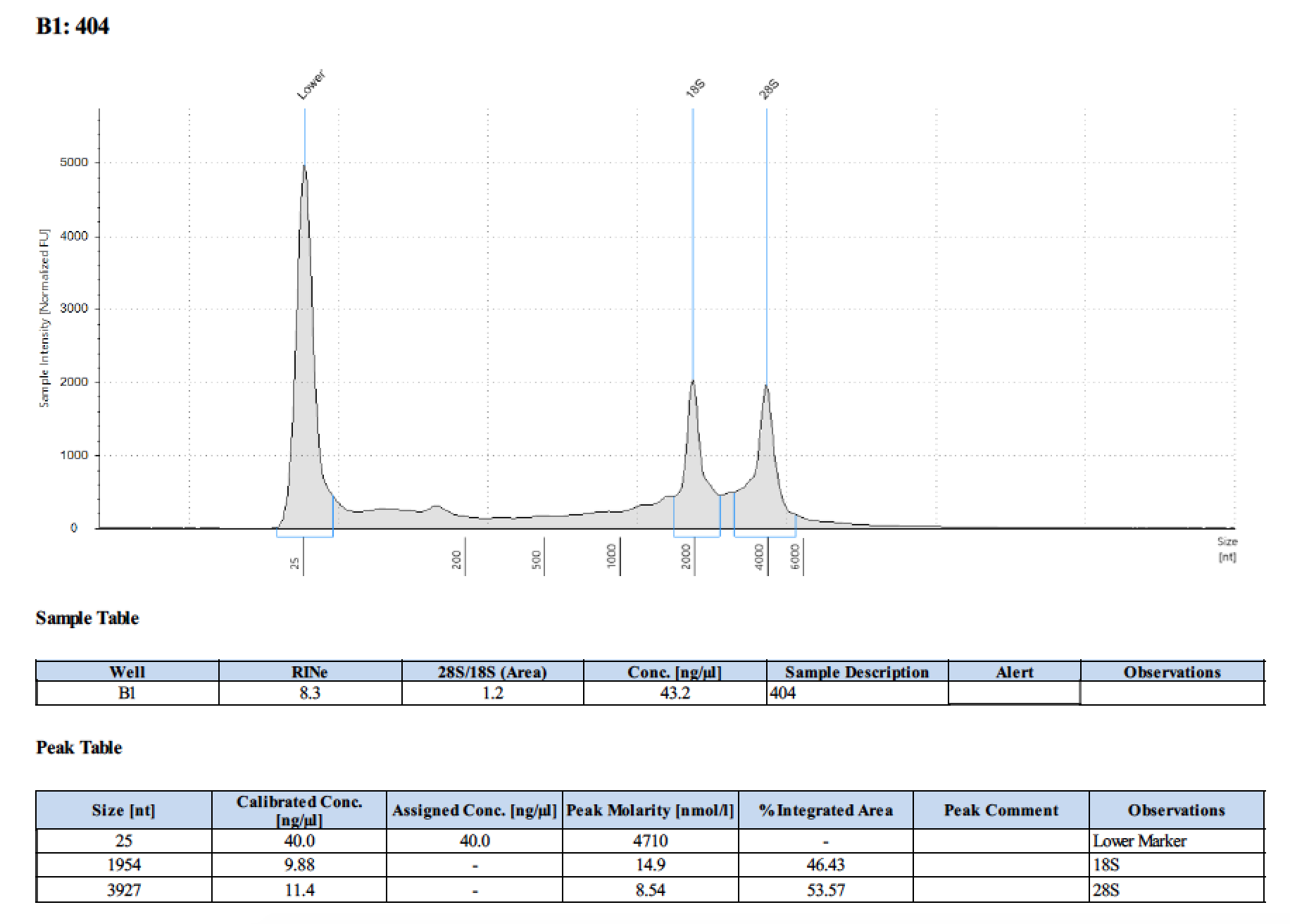
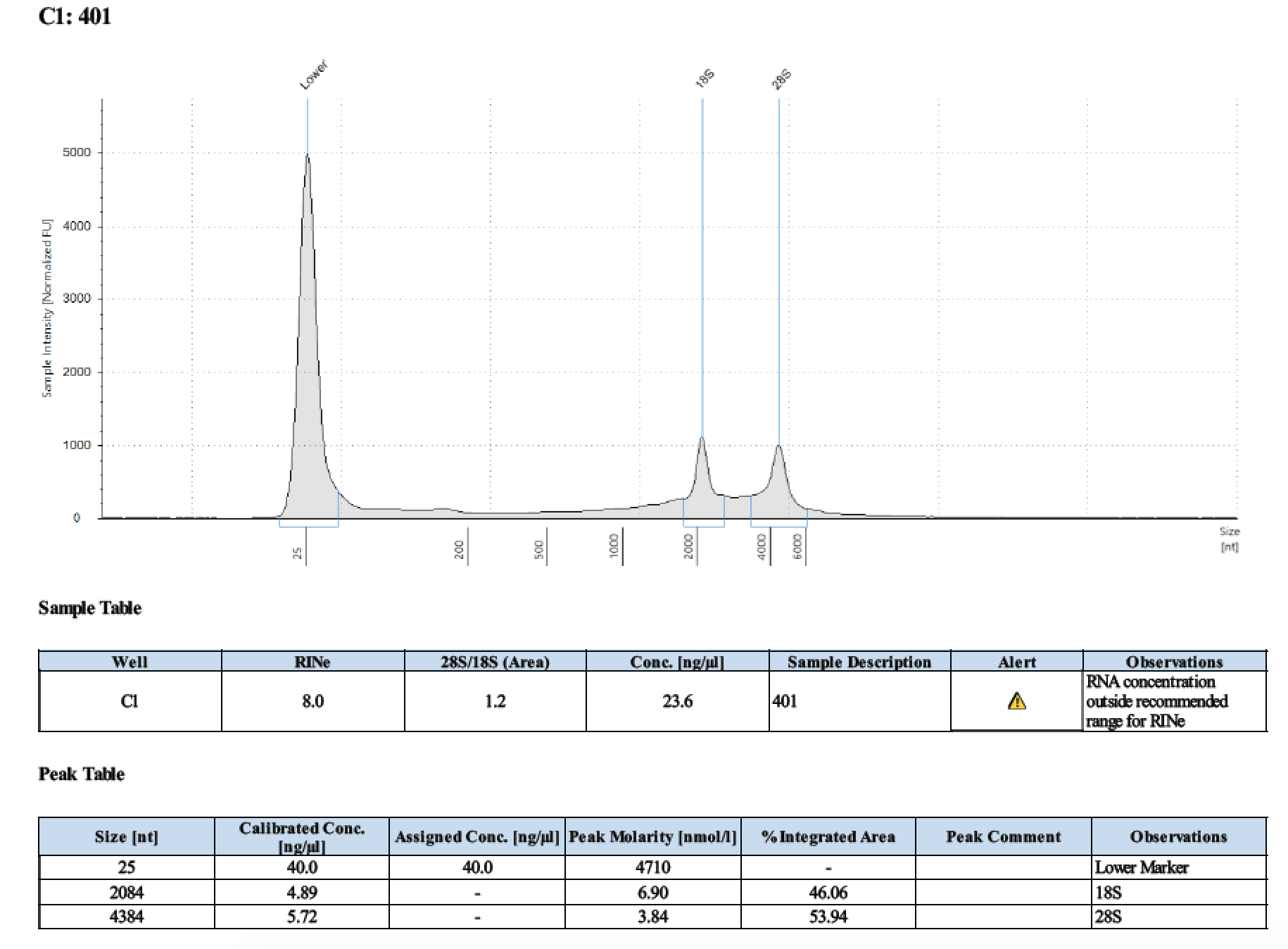
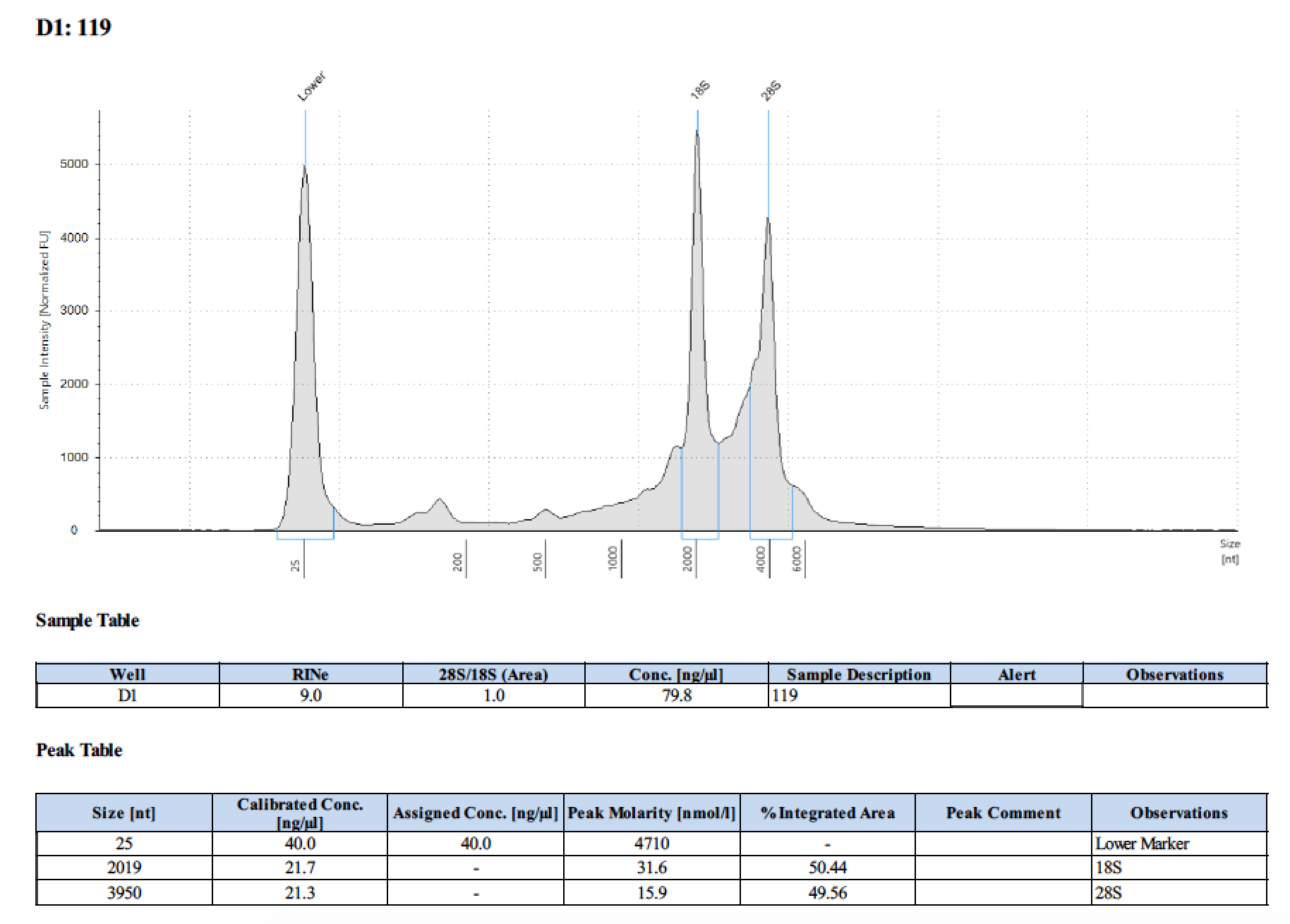
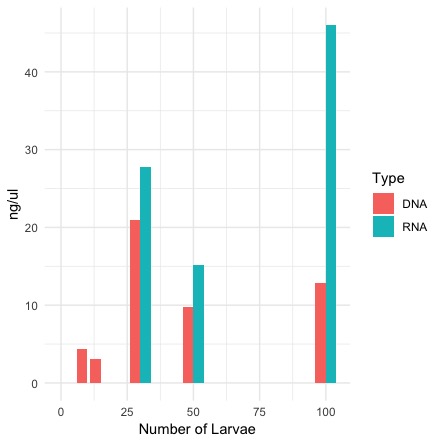
Graph of DNA and RNA together by number of larvae
R code for the graph
QubitValues <- read.csv("larvae.csv", header = TRUE)
library(ggplot2)
ggplot()+
geom_bar(data=QubitValues, aes(x=Number, y=ng, fill=Type, width=8), stat = "identity", position= "dodge") +
theme_minimal() + ylab("ng/ul") + xlab("Number of Larvae")
Written on March 8, 2019
